Hemolysis
Hemolysis FAQ
What is Hemolysis?
Hemolysis is the breakdown of red blood cells (RBC), resulting in the release of hemoglobin into blood plasma. Hemolysis can be a consequence of high fluid shear stresses as RBCs pass through narrow openings in devices such as mechanical circulatory support (MCS).
As a result, MCS devices are designed to minimize hemolysis and maximize blood compatibility.
RBCs traveling through unobstructed regions, such as regions away from the cannula wall, travel at higher speeds compared to RBCs in contact with the cannula wall. This difference in speed at which RBCs move past each other results in high shear force. When the shear force exceeds a critical level hemolysis results.1,2 Both the magnitude and duration of exposure to high shear stress may contribute to hemolysis.2
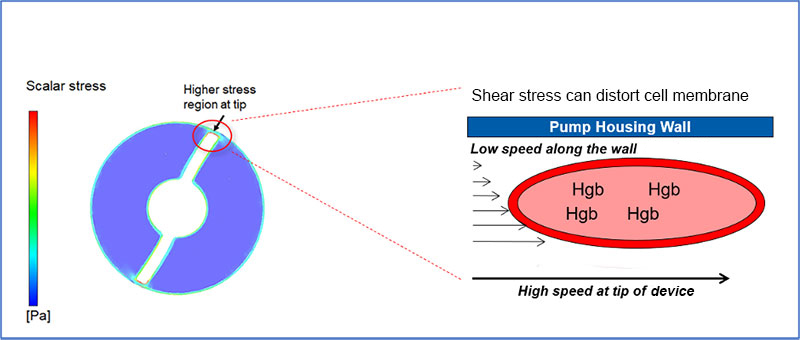
How is Hemolysis Measured?
Hemolysis is measured by detecting biomarkers representing release of red blood cells’ chemical contents such as plasma-free hemoglobin (PFH), lactate dehydrogenase (LDH), haptoglobin, indirect bilirubin, or by the presence of clinical signs such as hemoglobinuria. However, the sensitivity and specificity of the different markers for measurement of hemolysis vary widely. For example: LDH level can increase in response to any type of tissue injury and is frequently elevated in myocardial infarction and cardiogenic shock (AMICS). Therefore, LDH is not a specific hemolytic marker and should not be used in isolation to assess for hemolysis in AMICS.
There are currently no published recommendations for measurement of hemolysis in patients treated with acute mechanical circulatory (AMCS) devices. Since LDH should not be used to measure hemolysis in AMICS, the commonly used definition for hemolysis assessment with AMCS devices is PFH > 40 mg/dL on more than 2 measurements taken at least 8 hours apart.10
The INTERMACS definitions (summarized below) were developed for durable ventricular assist devices (VADs) and use PFH levels assessed 72 hours after VAD placement to distinguish hemolysis resulting from operative procedures from that resulting from the durable VAD itself. The INTERMACS definition may not be appropriate for MCS patients on support for less than 72 hours, and in particular AMICS patients who may have elevated LDH for reasons other than hemolysis.
Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) defines hemolysis as:3
Minor Hemolysis: PFH greater than 20 mg/dL or a serum lactate dehydrogenase (LDH) level greater than two and one-half times (2.5x) the upper limits of the normal (ULN) range at the implanting center occurring after the first 72 hours post-implant in the absence of clinical symptoms or findings of hemolysis or abnormal pump function.
Major Hemolysis: PFH value greater than 20 mg/dl or a serum lactate dehydrogenase (LDH) level greater than two and one-half times (2.5x) the ULN at the implanting center occurring after the first 72 hours post-implant and associated with clinical symptoms or findings of hemolysis or abnormal pump function. Major hemolysis requires the presence of one or more of the following conditions:
- Hemoglobinuria (“tea-colored urine”)
- Anemia (decrease in hematocrit or hemoglobin level that is out of proportion to levels explainable by chronic illness or usual post-VAD state)
- Hyperbilirubinemia (total bilirubin above 2 mg/dL, with predominately indirect component)
- Pump malfunction and/or abnormal pump parameters
How is Impella Designed to Maximize Blood Compatibility?
Shear stresses are determined by the speed of flow and geometry within the MCS device. Several engineering elements have been incorporated in Impella’s design and manufacturing to promote blood compatibility. The maximum tip speed of the impeller for all Impella devices (irrespective of pump size, and revolutions per minute (RPM)) has been limited to 10 m/s, ensuring that the shear stress generated is well below 400 Pa (shear stress threshold of normal RBCs).2,4 In addition, flow channels, gaps and the surfaces of the pump are optimized to reduce damage to RBCs and any blood component in general.
The amount of red blood cell damage is determined in vitro by assessing modified index of hemolysis (MIH), according to American Society of Testing and Materials (ASTM) standards.5 MIH is a ratio of RBCs destroyed during passage through the system to the total number of RBCs through the system.
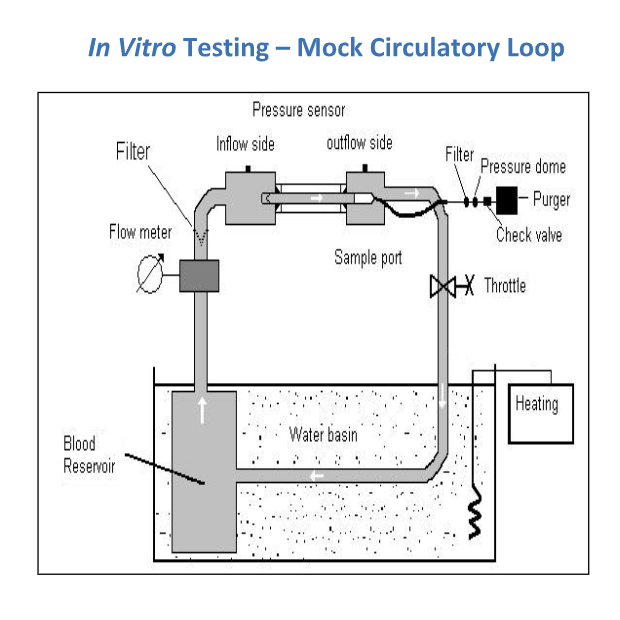
Impella heart pumps are tested in vitro using a mock circulation loop with porcine blood maintained at 37 ⁰C for 3-6 hours. MIH is calculated from the slope of the PFH concentration versus time plot. The mean MIH of Impella heart pumps is about 5, well below the MIH upper limit of 20 for use of blood pumps in clinical setting.5
What Factors Can Contribute to Hemolysis in the Clinical Setting?
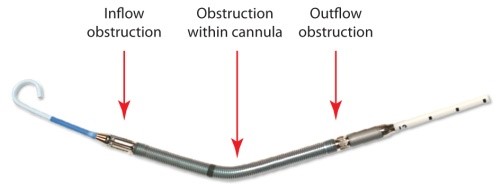
Obstruction to blood flow due to improper device placement is the most likely cause of hemolysis in the clinical setting. Hemolysis can occur due to interference to blood flow at the inlet, through the pump, and outlet of the pump.
If the inflow is obstructed due to suboptimal Impella positioning (for example: inflow position in the posterior portion of the LV behind a papillary muscle causing inflow obstruction), or low blood volume, suction will occur. In suction, the flow rate is reduced, however cells will experience more exposure time to the higher shear stresses near the impeller unless the P-level is turned down.
Obstruction within the pump (for example: fiber or clot on impeller) creates flow disturbances near the impeller which may result in high shear and hemolysis.
If the outflow windows are obstructed by the aortic valve leaflets, flow disturbances and turbulence will occur, thus increasing shear stresses and hemolysis.
Impella heart pumps have been tested under simulated conditions of inflow and outflow obstruction. Conditions mimicking inlet obstruction (continuous or diastolic suction) resulted in an increase in MIH by 2.5 times while conditions of outlet obstruction increased MIH by 6 times. These results suggest that high levels of hemolysis with Impella more likely result from conditions blocking the Impella outlet. Hence, proper maintenance of optimal positioning of Impella devices is key to preventing the occurrence of hemolysis.15
Obstructions to Blood Flow May Cause Hemolysis
Proper Impella Position to Reduce Hemolysis
- Unobstructed Inflow
- Approx. 3.5 cm distal to aortic valve (Approx. 5 cm for the Impella 5.5® with SmartAssist®)
- Free from anterior mitral leaflet
- Free from sub-annular structures
- Outflow well above the aortic valve
- Stable position that does not migrate
How Does Impella’s Blood Compatibility Compare to Other Heart Pumps in Pre-Clinical/Bench Testing?
Impella devices have equivalent, or lower rates of hemolysis compared to other heart pumps in bench testing.
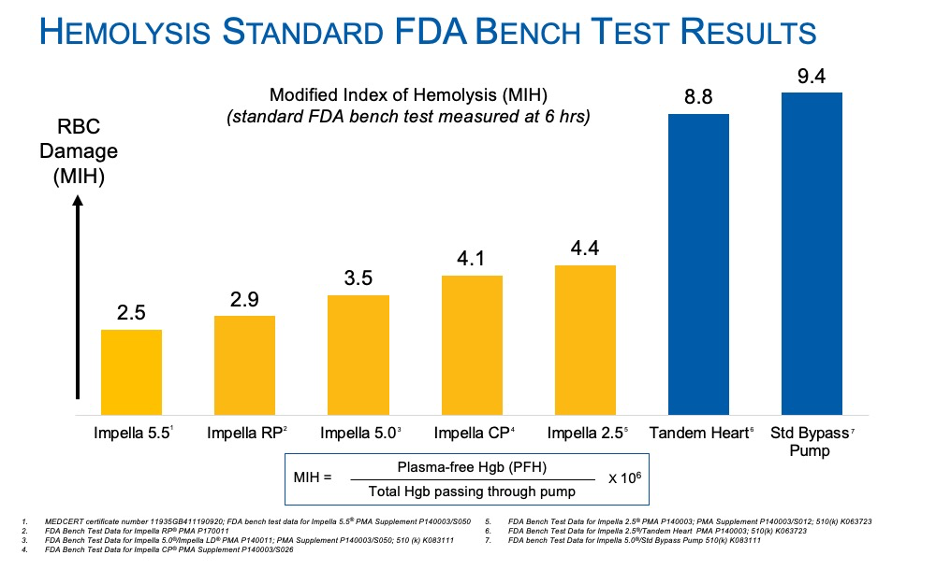
Bench testing is performed according to ASTM F1841, which is the standard practice for assessment of hemolysis in continuous flow blood pumps. All pumps must pass this test and submit to the FDA before being cleared or approved in the U.S. The Modified Index of Hemolysis (MIH) graph displays a comparative characterization of heart pumps under this setting. The test is standardized at six hours and MIH is used as a comparator.
Results show Impella devices result in the least amount of plasma free hemoglobin and lowest MIH value after six hours of use. Impella had an MIH value of 2.5-4.4, which can be considered very similar and with plenty of safety margin, regardless of RPM.
In all cases, the Impella RPM translates into an impeller tip speed of < 10 m/sec; therefore, red blood cells traveling through an Impella pump cannot distinguish between Impella 2.5®, Impella CP, or Impella 5.5 – the shear stresses are the same. Clinical differences between the Impella pumps are due to positioning and suction.
TandemHeart had at an MIH value of 8.9 and the industry standard bypass pump was 9.4 MIH. Both were more than double the MIH of Impella.15
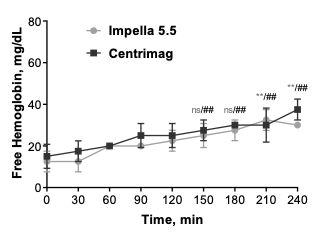
Impella 5.5® with SmartAssist® versus CentriMag™: Head-to-Head Comparison of Device Hemocompatibility
Marvin Slepian, MD, compares the hemocompatibility of Impella 5.5 to Centrimag discussing hemolysis, platelet activation, microparticle generation, and vWF alteration.
An additional in vitro, head-to-head comparison was performed at the Slepian Lab between the Impella 5.5 with SmartAssist and CentriMag.15 Blood compatibility was found to be comparable for both pumps (MIH <2) despite the Impella 5.5 RPM >> CentriMag RPM. Tip speed determines damage to the blood cell not RPM, and both pumps are gentle to red blood cells. Watch the video above to learn more. Additionally, Impella 5.5 with SmartAssist was associated with less platelet damage and generated less procoagulant microparticles than CentriMag.
How Does Impella’s Blood Compatibility Compare to Other Heart Pumps in Clinical Settings?
Impella heart pumps have lower rates of hemolysis compared to ECMO based on published clinical studies.
Currently, there are no published recommendations for hemolysis assessment in patients treated with AMCS devices. Hence, the definition of hemolysis varies widely between studies of AMCS devices, making clinical comparisons challenging. Based on surveillance of published literature, the reported rates of hemolysis with Impella and other MCS devices are as follows:
| MCS Device | Impella 2.5 |
| Hemolysis (%) | 0.16% - 10.3% |
| Study Type | RCT and observational studies |
| MCS Device | Impella CP |
| Hemolysis (%) | 8% - 11.9% |
| Study Type | RCT and observational studies |
| MCS Device | Impella RP® |
| Hemolysis (%) | 7% - 13.3% |
| Study Type | Observational studies |
| MCS Device | IABP |
| Hemolysis (%) | 7.1% |
| Study Type | RCT |
| MCS Device | TandemHeart |
| Hemolysis (%) | 5.2% |
| Study Type | RCT |
| MCS Device | ECMO |
| Hemolysis (%) | 18% |
| Study Type | Meta-analysis of observational studies |
Troubleshooting Suspected Hemolysis in Patients Supported with Impella Heart Pump
Jessica Page, Global Impella Trainer, reviews the clinical signs and symptoms of hemolysis.
References
- Leverett, L.B., et al. (1972). Biophys J, 12(3), 257–273.
- Baskurt, O.K., et al. (2012). Engineering. 5, 8-10.
- Interagency Registry for Mechanically Assisted Circulatory Support, National Heart Lung and Blood Institute. INTERMACS Adverse Events Definitions: Adult and Pediatric patients. Available at http://www.uab.edu/medicine/intermacs/images/protocol_5.0/appendix_a/AE-Definitions-Final-02-4-2016.docx. Published May 15, 2013. Accessed July 22, 2018.
- Lee, S.S., et al. (2004). Korea-Australia Rheology Journal, 16(3), 141-146.
- ASTM F 1841-97, ASTM 1830-97, American Society for Testing and Materials, Subcommittee F04.30 Annual Book of ASTM Standards (Vol 13.01.), ASTM, West Conshohocken, PA (1998).
- CentriMag/Industry standard data – Levitronix Website
- FDA Bench Test Data for Impella 5.0/LD PMA P140003
- FDA Bench Test Data for Impella CP PMA Supplement P140003/S004
- FDA Bench Test Data for Impella 2.5 PMA P140003
- FDA Comparative Bench Test Data for Impella 2.5 510(k) Clearance K063723
- FDA Bench Test Data for Impella RP PMA P170011
- Dixon, S.R., et al. (2009), 2(2), 91-96.
- O'Neill, W.W., et al. (2012). Circulation, 126(14), 1717-1727.
- Cohen, M.G., et al. (2015). Am Heart J, 170, 872-879.
- Roberts, N., et al. (2020). Artificial Organs, 1-9.
- Roka-Moiia, Y., et al. (2020). ASAIO, 66(10), 1142-1151.
IMP-387